Lactase Persistence
Humans are relatively unique among mammal species in that some of their adult population retains the ability to digest lactose, a sugar found in milk. This ability is the result of a mutation that occurred thousands of years ago, and that must have conferred some adaptive advantage to those with the trait. This module will explore how, where, and why this evolutionary event took place.
PowerPoint Slides
Question Guide
Games and Sims
NCCSTS Case Study

Molecular Genetics
Most mammals, including 65% of humans, cannot digest lactose as adults. Lactase persistence, the opposite of lactose intolerance, is the result of an evolutionarily conserved mutation in the regulatory mechanisms of lactase-mRNA production. A SNP (single nucleotide polymorphism) in the binding site of one of lactase’s transcription factors is associated with the continuation of lactase production into adulthood.
Biosynthesis of Lactase
 The human lactase gene (LCT) is a 55 kilo-base pair segment of the second chromosome. It contains 17 exons. LCT is transcribed into Lactase-mRNA by RNA polymerase, and Lactase-mRNA is translated by a membrane-bound ribosome into a polypeptide called pre-pro-lactase. During translation, the 1,927 long amino acid sequence is fed into the ER (endoplasmic reticulum), but remains anchored in the lipid bilayer of the ER membrane. Several subunits of pre-pro-lactase are cleaved off as the enzyme is processed into its mature form. The immature protein is dimerized (attached to another copy of itself) within the ER. Then a transport vesicle containing pro-lactase blebs off the ER and travels to fuse with the Golgi Apparatus. Once within the Golgi Apparatus, the “pro” subunit prevents degradation and ensures proper folding of lactase into its mature quaternary structure before it is cleaved off. Finally, a vesicle containing mature lactase travels from the Golgi Apparatus to fuse with the external brush border membrane of epithelial cell. Here, the enzyme will carry out its function of breaking down dietary lactose.
The human lactase gene (LCT) is a 55 kilo-base pair segment of the second chromosome. It contains 17 exons. LCT is transcribed into Lactase-mRNA by RNA polymerase, and Lactase-mRNA is translated by a membrane-bound ribosome into a polypeptide called pre-pro-lactase. During translation, the 1,927 long amino acid sequence is fed into the ER (endoplasmic reticulum), but remains anchored in the lipid bilayer of the ER membrane. Several subunits of pre-pro-lactase are cleaved off as the enzyme is processed into its mature form. The immature protein is dimerized (attached to another copy of itself) within the ER. Then a transport vesicle containing pro-lactase blebs off the ER and travels to fuse with the Golgi Apparatus. Once within the Golgi Apparatus, the “pro” subunit prevents degradation and ensures proper folding of lactase into its mature quaternary structure before it is cleaved off. Finally, a vesicle containing mature lactase travels from the Golgi Apparatus to fuse with the external brush border membrane of epithelial cell. Here, the enzyme will carry out its function of breaking down dietary lactose.
Regulation of Lactase Synthesis
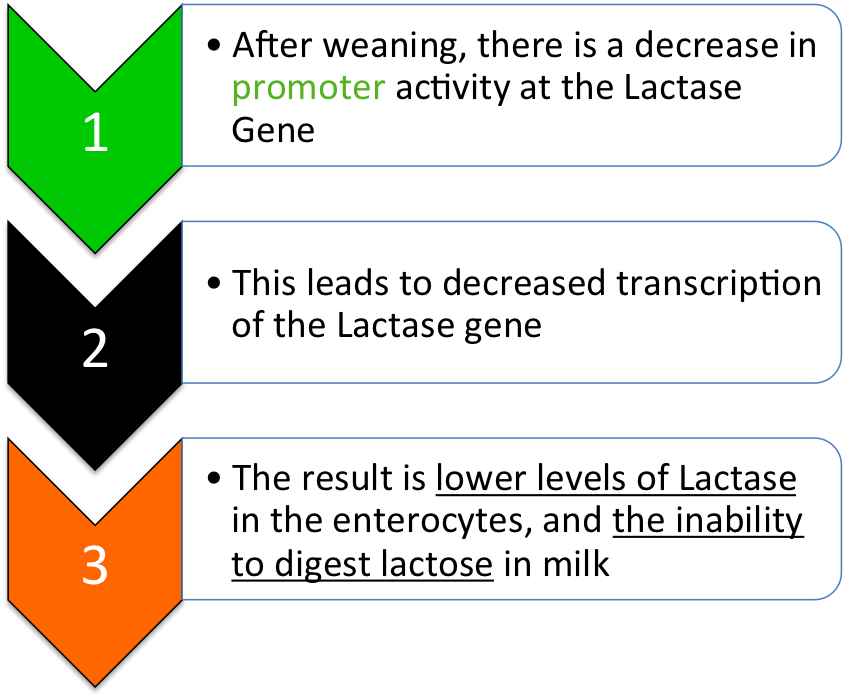
The regulation of lactase synthesis over developmental time is the factor that separates lactase persistent from non-persistent individuals. In most mammals, including 65% of humans, the level of lactase-mRNA found in the enterocytes is greatly decreased over the years after weaning. This is because mammals don’t typically consume milk in adulthood, so the production of enzymes to help digest milk is unnecessary and therefore energetically wasteful at a cellular level. Age-dependent lactase regulation of this sort occurs at transcription.
Transcription Factors
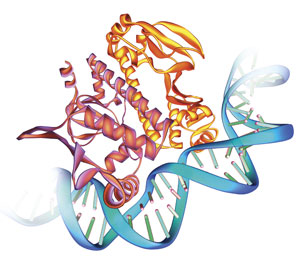 A transcription factor (TF) is a protein that binds to a specific segment of DNA and influences a gene’s transcription frequency. Once bound to DNA, a TF either attracts or repels the molecular machinery necessary for transcription. TFs can even attract other transcription factors to form large transcription complexes.
A transcription factor (TF) is a protein that binds to a specific segment of DNA and influences a gene’s transcription frequency. Once bound to DNA, a TF either attracts or repels the molecular machinery necessary for transcription. TFs can even attract other transcription factors to form large transcription complexes.
Special TFs called “activators” bind to specific enhancer sites on the DNA; these activators are helpful in initiating transcription by binding to RNA Polymerase and other enzymes used in transcription. This type of TF therefore increases the expression of a gene.
Other TFs influence the probability and frequency of transcription by binding to transcription factors at enhancer sites. Enhancer sites may be far away from the start of a gene, but the DNA loops around permitting the enhancers to come into contact with the transcription complex. This increases the frequency of transcription of the gene and, by extension, increases the expression of a gene.
A series of transcription factors have been identified that regulate the amount of lactase-mRNA an intestinal epithelial cell produces over the course of its life. These transcription factors bind to the DNA about 14,000 base pairs upstream of the lactase gene, within the introns (non-protein-coding regions) of an upstream gene, MCM6. Much of the research concerning the evolution of lactase persistence in humans focuses not on mutations in the lactase LCT gene, but rather on mutations in these enhancers within the introns of the MCM6 gene.
Single Nucleotide Polymorphism
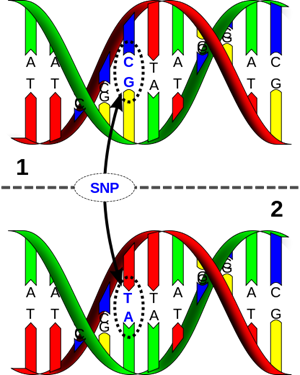 If a nucleotide substitution mutation achieves a frequency of 1% in a population over time, it is considered a “single nucleotide polymorphism”, or SNP.
If a nucleotide substitution mutation achieves a frequency of 1% in a population over time, it is considered a “single nucleotide polymorphism”, or SNP.
The type of mutation that is studied in the context of lactase persistence evolution is a called a single nucleotide polymorphism, or SNP. A SNP is a mutation where only one nucleotide in a sequence is changed.
Several SNPs are associated with lactase persistence, all of which are thought to increase or decrease a transcription factor’s ability to bind to DNA at that site. This binding affinity influences the likelihood that transcription factors will attach to the DNA and either repel or attract RNA polymerase.
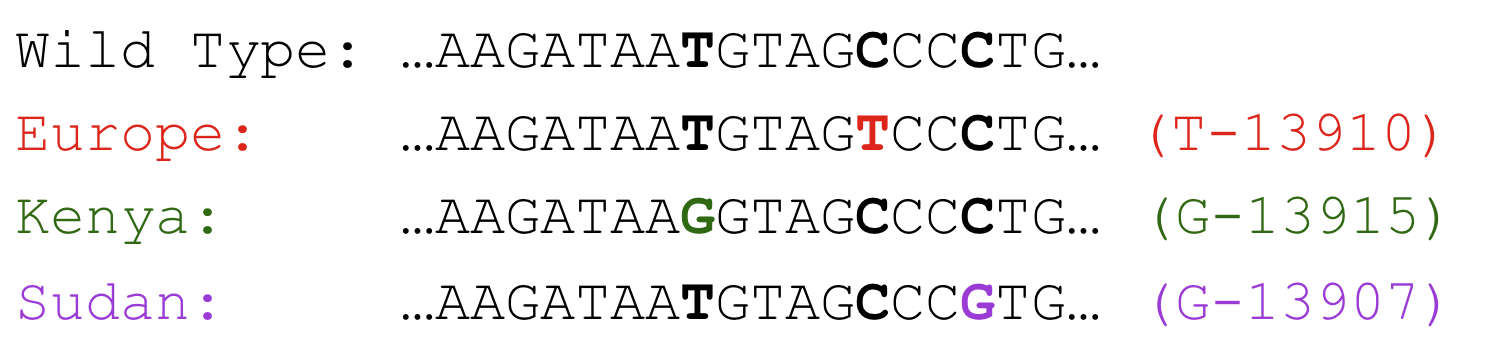
In one well-studied SNP, 13910 bp upstream of the lactase gene, a Thymine base has been substituted into the DNA sequence in the place of a Cytosine base. This mutation (T instead of C) increases the binding affinity of a transcription factor called Oct-1, which acts as an activator. It has been shown to increase transcription complex binding to the promoter, and therefore increase production of lactase-mRNA.
Wildtype Pathway (Lactose Intolerance):
Mutant Pathway (Lactase Persistence): 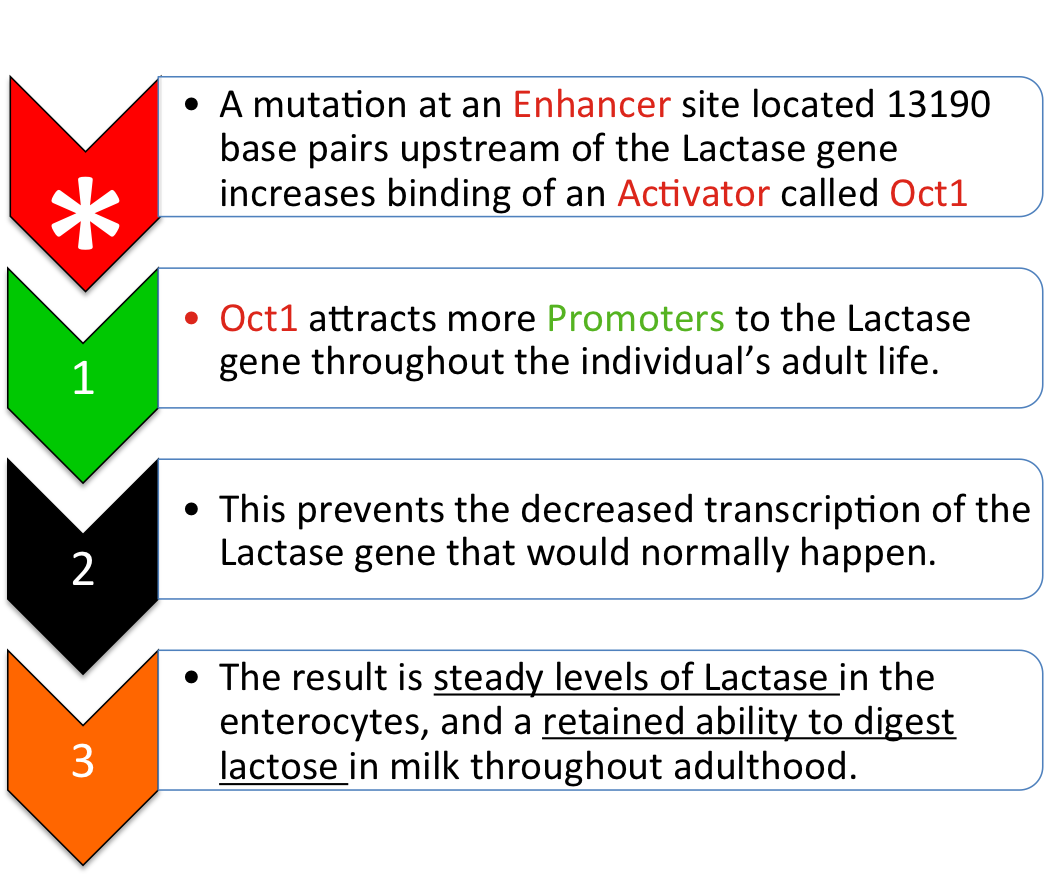
Cell Biology
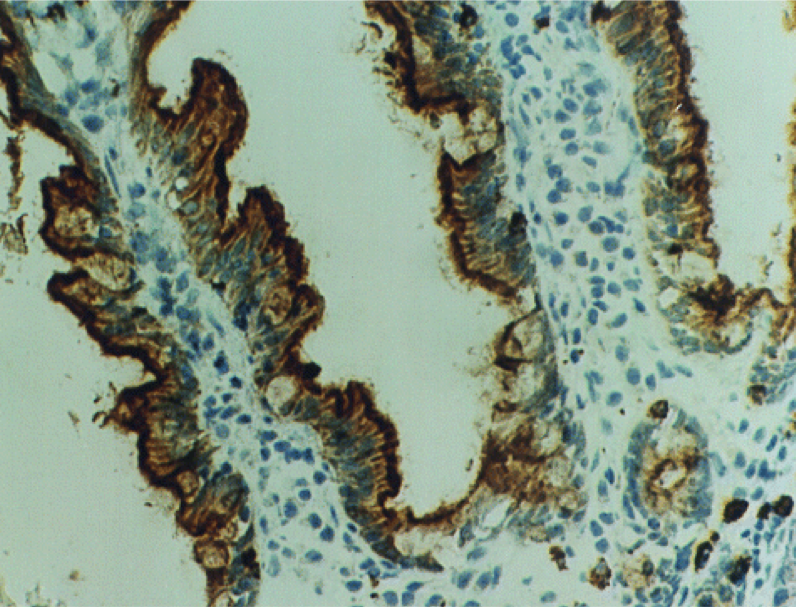 The enzyme Lactase is a transmembrane protein in intestinal epithelial cells, or enterocytes. Its function is to break lactose into its two constituent sugars: glucose and galactose. These constituent sugars can then be used in ATP manufacturing and other cellular processes.
The enzyme Lactase is a transmembrane protein in intestinal epithelial cells, or enterocytes. Its function is to break lactose into its two constituent sugars: glucose and galactose. These constituent sugars can then be used in ATP manufacturing and other cellular processes.
How does Lactase work?
Lactase is a transmembrane protein located in the lipid bilayer membrane such that its active sites extend into the lumen of the intestine. When the enzyme lactase binds to the disaccharide lactose, its active sites cleave lactose into its two constituent sugars: glucose and galactose. Glucose and galactose are then free to be absorbed through the intestinal epithelial cells and transported into the bloodstream.
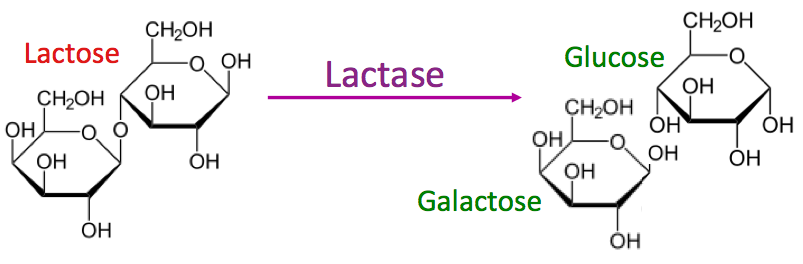
Absorption of Glucose and Galactose
After lactase is broken down into glucose and galactose, the glucose transporters SGLT1 and GLUT2 facilitate the diffusion of glucose and galactose through the enterocyte and into the bloodstream. This process is powered by the diffusion of these molecules down their concentration gradient: there is a higher concentration of glucose and galactose in the lumen of the intestine than in the enterocyte cell body, and there is a higher concentration of glucose and galactose in the enterocyte cell body than in the blood.
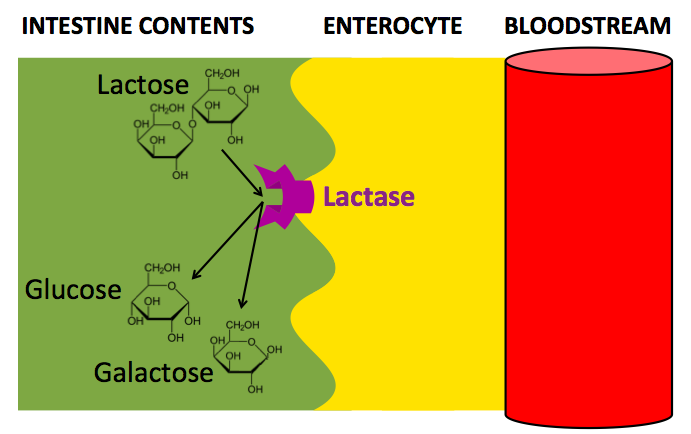
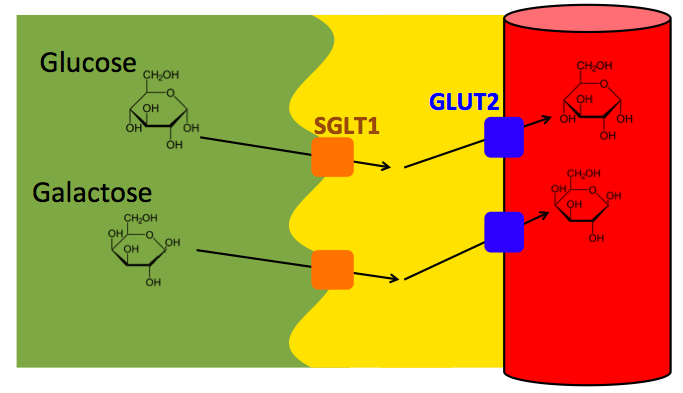
In the above diagrams: Lactase is a transmembrane protein on the interior border of the enterocyte. When lactose from the intestine contents comes into contact with the active site of lactase, it is broken down into glucose and galactose. The SGLT1 transmembrane protein (Sodium-Glucose Linked Transporter 1), transports glucose or galactose via facilitated diffusion from the intestine into the enterocyte. Then GLUT2 (Glucose Transporter 2) transports glucose or galactose via diffusion from the enterocyte to the bloodstream.
The Cell Biology of Lactose Intolerance
 If lactose is not broken down in the small intestine, as is the case in lactose intolerant individuals, it is passed into the large intestine. This leads to several problems that are characteristic of lactose intolerance. The high concentration of lactose in the large intestine creates an osmotic gradient, which causes large volumes of water to move from the blood into the gut as the system begins to equalize the solute concentrations on either side of the intestine wall. This excess water leads to cramping and diarrhea, which causes pain and can lead to dehydration.
If lactose is not broken down in the small intestine, as is the case in lactose intolerant individuals, it is passed into the large intestine. This leads to several problems that are characteristic of lactose intolerance. The high concentration of lactose in the large intestine creates an osmotic gradient, which causes large volumes of water to move from the blood into the gut as the system begins to equalize the solute concentrations on either side of the intestine wall. This excess water leads to cramping and diarrhea, which causes pain and can lead to dehydration.
Furthermore, when lactose is not broken down by lactase in the small intestine it can be consumed by bacteria that live in the large intestine. Many of these bacteria use the process of sugar fermentation to produce ATP. The fermentation process produces large amounts of gaseous by-products, such as methane, carbon dioxide, and hydrogen. This leads to gas build-up in the gut, resulting in cramping and flatulence.
Lactase Regulation
Almost all known mammals – and 65% of humans – experience a decrease in lactase biosynthesis in the years after weaning. However, the remaining 35% of humans continue to produce lactase after weaning, and are therefore able to continue to consume milk and other dairy products into adulthood. In the case of lactase persistence, there is a continued production of lactase at high levels throughout adulthood.
Why is the production of lactase regulated in the first place? Why not just produce lactase in the enterocytes of all adult mammals? The answer is a matter of cellular energetics. Most mammals (humans notwithstanding) do not consume milk after they have been weaned. Therefore, the energy invested in biosynthesizing lactase is an unnecessary expenditure. This may seem like a very small amount of energy, but remember that everything that occurs in an organism is directed at the cellular level. Cellular energetics are in fact a major evolutionary pressure, and have shaped the evolution of the inner workings of cells since life began.
Biogeography and Anthropology
The evolution of lactase persistence is thought to have occurred in Middle Eastern dairying populations during the Neolithic era. Prior to this, ancient humans were lactose intolerant like all other mammals. In fact, lactose intolerance persists in most human populations today. However, once the lactase persistence trait evolved, strong selective pressure allowed it spread throughout ancient human populations.

The Neolithic Era
When did humans evolve the ability to digest milk far into adulthood? Through genetic analysis, scientists estimate that this evolutionary step occurred between 5,000 and 7,000 years ago, in the Neolithic Era (Itan, 2009). During this time, humans were making the major transition from hunting and gathering to farming and herding. This offered increased food security and promoted technological innovation as farmers and herders learned new ways to manipulate their environment and resources (Mummert, 2011).
Pastoralism, the cultural practice of milking livestock (such as goats, sheep, cows, and camels), was an innovation of the Neolithic Revolution. The Biocultural Coevolution theory proposes that pastoralism and lactase persistence coevolved. This means that they arose around the same time, and both changes were reinforced by the advantages of the other.
Evolutionary Advantages
There are significant nutritional benefits of consuming dairy products. Milk contains essential molecules such as water, sugar, fat, protein, and vitamins. Although Neolithic humans could obtain these nutrients from the meat of their livestock, the ability to obtain these nutrients without killing their animals would have afforded them a great increase in efficiency and stainability. Additionally, dairy products could have been a life-saving source of calories during times of famine. Within a pastoralist culture, an individual with lactase persistence would have a distinct advantage in terms of nutrient acquisition.
Migration
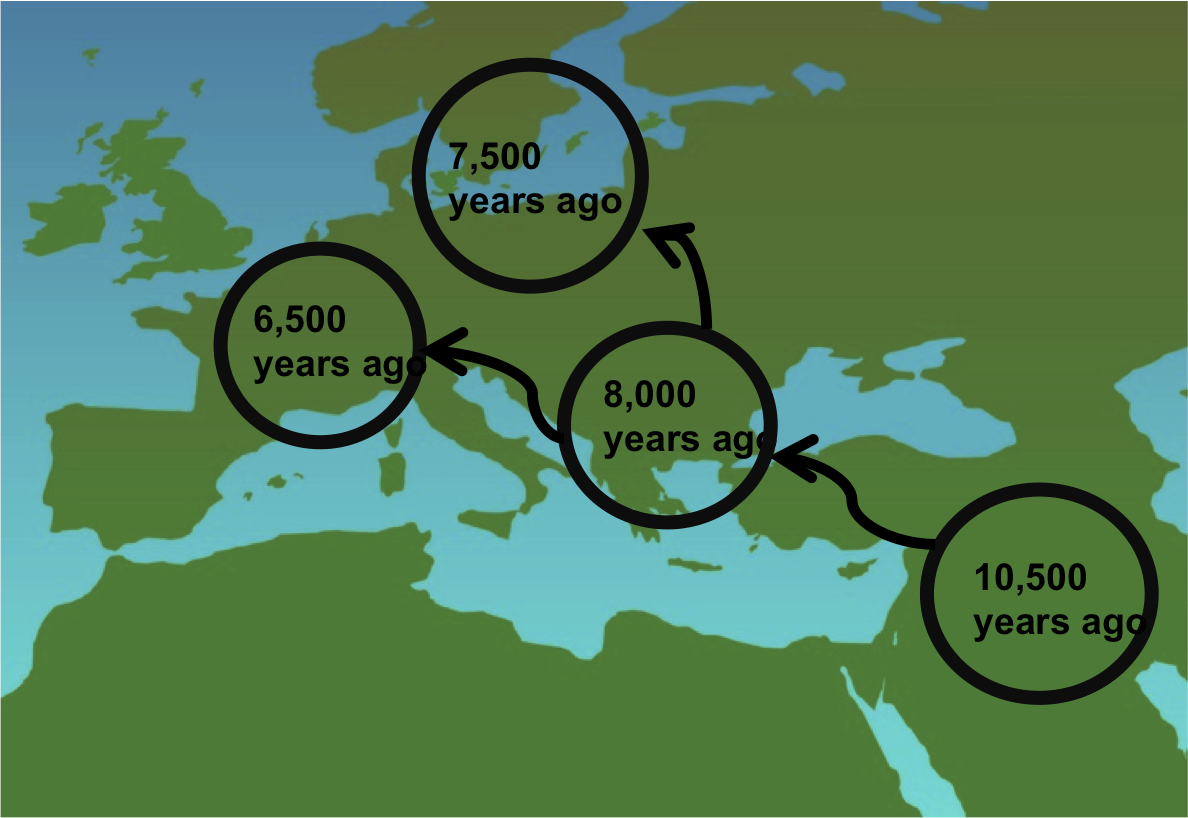
How did Lactase Persistence spread? Simply stated, it spread with the cultural practice of milking livestock, or Pastoralism. The earliest evidence of pastoralism, 10,500 year old bones of juvenile cattle, was found in the Middle East. It is thought that this practice spread through migration because the remains of the earliest domesticated cattle in Greece and the Balkan States (8,000 years old) were more closely related to the cattle from the Middle East than the wild cattle found in Europe at the time. This indicates that migrants from the Middle East brought their cattle with them. Because Middle Eastern cattle herders had more advanced food technology, they easily out-competed the local hunter-gatherers they encountered in Central and Northern Europe.
Milk vs. Cheese
 Although it is estimated that the prevalence of lactase persistence greatly increased in Europe around 5,000 to 7,000 years ago, anthropological evidence, like bones of juvenile livestock and ancient pottery remains, place the cultural adoption of dairying as far back as 12,000 years ago. This suggests that for several thousand years, humans were milking sheep, goats, and cows despite the inability to digest milk.
Although it is estimated that the prevalence of lactase persistence greatly increased in Europe around 5,000 to 7,000 years ago, anthropological evidence, like bones of juvenile livestock and ancient pottery remains, place the cultural adoption of dairying as far back as 12,000 years ago. This suggests that for several thousand years, humans were milking sheep, goats, and cows despite the inability to digest milk.
Shards of ancient ceramic sieves provide some clues as to how this was possible for early farmers and herders. Many Paleolithic and Neolithic cultures practiced the storage and transport of food and water in animal skins and intestines. When milk was introduced to the Neolithic diet in the Middle East, it was likely stored in an inflated cow stomach, resulting in the separation of the curds and whey. By using ceramic sieves to strain the curds and whey and ferment these diary products into cheese, they were able to greatly reduce the level of lactose in their dairy products, while still retaining the nutritional benefits of milk. (Salque, 2013).
Convergent Evolution
Convergent evolution occurs when separate populations independently evolve the same trait. This is often due to both populations experiencing similar selective pressures that help to fix a new trait in a population once it arises through random mutation. In the case of lactase, the selective force is benefit accrued from milk consumption by human adults.
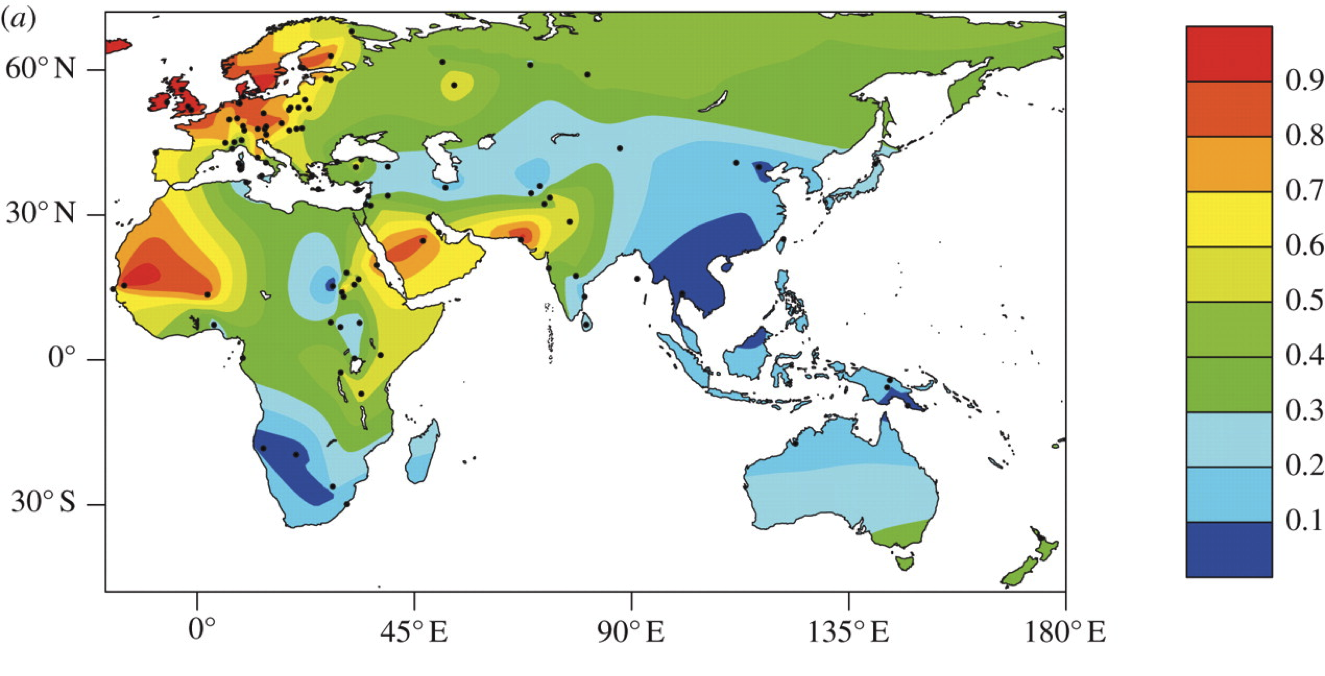
In Africa, lactase persistence evolved independently from the European lineage. The mutation(s) responsible for lactase persistence are different, but the effect is the same.
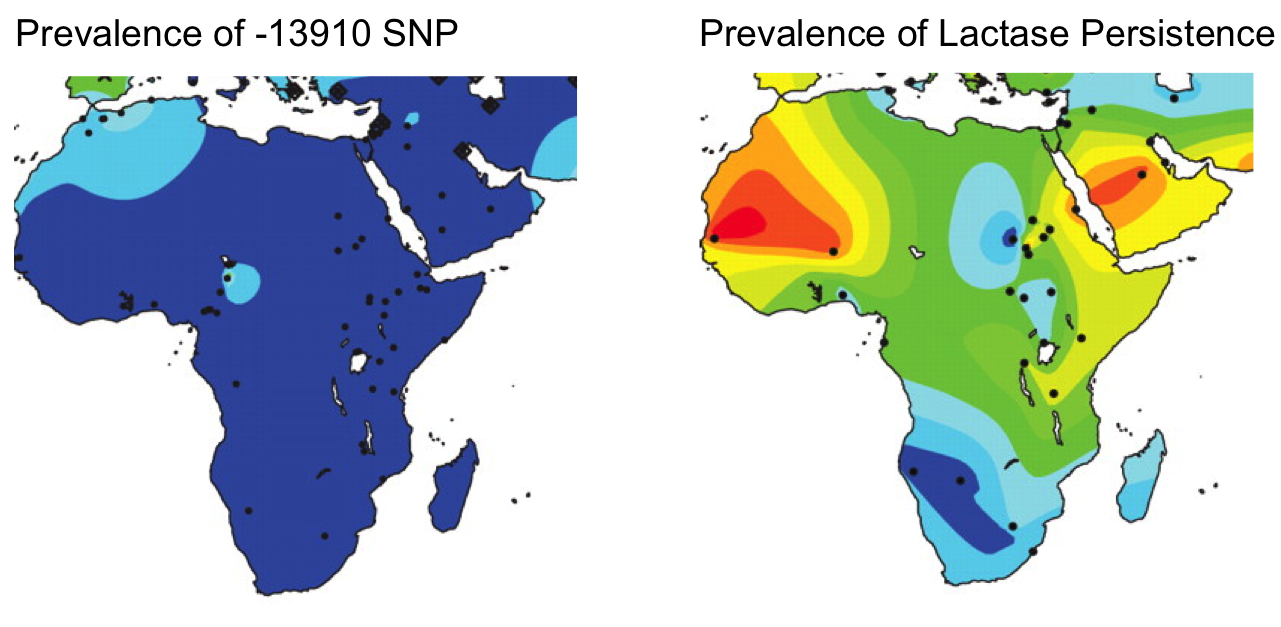
Neolithic humans in Africa experienced similar selective pressures to adopt pastoralism and evolve lactase persistence. Through slightly different mutations, G-13915 and G-13907, different peoples in Kenya and Sudan evolved lactase persistence via the same Oct1 transcription factor binding site.
A Special Case
The Somali people of Ethiopia are an example of a modern culture that have adopted a pastoralist way of life, drink milk as adults, and yet have not evolved the lactase persistence trait. How is it possible that adults regularly consume fresh milk from their cows without the negative consequences? The answer is in their large intestines: individuals in this culture have been found to posses unique colonic bacterial populations that metabolize lactose in such a way that does not cause gastrointestinal distress (Ingram, 2008).
