Breast Cancer
 Breast Cancer
Breast Cancer
Approximately 1 in 8 women will have a diagnosis of breast cancer over their lifetime. This case looks at what makes breast tissue so susceptible to cancer and examines particular risks, both environmental and genetic, that contribute to this high incidence. Breast epithelial cells receive growth signals via hormones as part of a normal menstrual cycle. Compared to cells in other tissues, they reproduce at a relatively high rate. This permits somatic mutations in breast tissue cells to occur and accumulate at a high rate, leaving breast tissue prone to cancer. Some germ line (inherited) mutations also contribute to the onset of breast cancer. The persistence of these mutations can potentially be explained as an evolutionary trade-off or via founder effects. In other cases, the onset of breast cancer can be explained by exposure to environmental factors, such as modern diets, cigarettes, and changes in reproductive patterns.
This case will introduce the mechanistic and evolutionary underpinnings of breast cancer. It will also explore how an evolutionary perspective can be utilized to improve the treatment of some cases of breast cancer.
Genetics
- Explain how DNA influences the expression of phenotypes and describe the series of biological processes that occur. Define the concept of gene variants
- Define mutation and explain the difference between somatic and germline mutations. Describe how mutations may lead to the development of cancer
- Describe the difference between proto-onco genes and tumor suppressor genes, and explain how variants of these genes may increase risk of cancer
Genes, genetic variation, and cancer
All expressed biological traits (phenotypes), including cancer, are influenced in part due to genetics. DNA inside the nucleus is transcribed into RNA, and RNA is translated by ribosomes in the cytoplasm into proteins. Each protein has a specific function that contributes to determine every trait expressed by an organism. This expressed trait is called a phenotype. Every individual harbors a unique sequence of DNA, called a genome, which consists of numerous genes. Within each gene, differences in the sequence of nucleotide base-pairs exist between individuals. These differences are called gene variants, or alleles. Since the nucleotide sequence ultimately determines the production of a protein, different gene variants sometimes result in proteins with different functions and thus different expressed traits.
When it comes to cancer, there are many proteins that function to keep cell proliferation in check and work to suppress tumors from forming. For example, some proteins initiate mitosis when a cell gets too old or when tissue growth and/or replacement is needed, and others check that DNA is properly replicated before mitosis begins. Therefore, if a gene variant exists that disrupts the function of one of these proteins, cancer can arise.
Mutations, the somatic mutation theory of cancer, and inherited cancer genes
Mutations are the origin of all novel genetic variation. Mutations are “mistakes” that randomly occur during the process of DNA replication and can thus arise every time a cell replicates. One theory of cancer onset is the somatic mutation theory. In this case, cells that make up tissue can escape normal cell cycle regulation by accumulating a number of mutations in key genes that code for proteins that function to keep cellular cooperation and replication in check. Somatic mutations are mutations that occur within any cell in the body other than gametes (i.e. sperm and egg cells), and therefore cannot be inherited. Since somatic mutations have the potential to occur each time a cell replicates, and thus accumulate through continuous aging and division of cells. In the case of breast cancer, breast tissue cells reproduce a relatively high rate compared to other cells in the body – thus making breast tissue prone to cancer.
Some rogue gene variants can be inherited, which can occur if a mutation arises in a gamete and continues to persist in a population over generations. This is why it is commonly said that someone has “inherited a gene for breast cancer”. Somewhere between 5 – 10% of breast cancers are related to disease-causing inherited gene variants. It has been hypothesized that these variants continue to persist in populations since many cancers, including breast cancer, do not emerge until a post-reproductive age (see Trade-offs).
HER2 and ERS1, proto-oncogenes
Proto-oncogenes are cell cycle “throttles”, the protein products of which are involved in kick-starting cell reproduction and DNA synthesis. Pathogenic variants are called oncogenes, which allow unchecked cell reproduction. The gene HER2 is an example of proto-oncogene that underlies many cases of breast cancer. HER2 is a member of the human epidermal growth factor family of genes. HER2 is located on chromosome 17 and has 30 coding regions which contain around 400 nucleotides. Mutations on promotor regions can increase the expression of this gene and are implicated in around 20-30% of invasive breast cancers. Some variants contain mutations are the exons of the gene, resulting in proteins with increased function. For example, a single point mutation at position 1963 where adenine is substituted for guanine results in a non-synonymous protein change. This means that the sequence of amnio acids that makes up the protein has changed, specifically the amino acid at position 655 changes from valine (GTC) to isoleucine (ATC). In females, this single change is associated with a 20-160% increased risk of developing breast cancer (Xie et al., 2000).
ERS1 It is located on chromosome 6 and encodes an estrogen receptor. Pathogenic variants play a role in osteoporosis, as well as breast, ovarian and endometrial cancer. Similar to HER2, variants that result in the overexpression of the gene or proteins with increased functioning will result in an increased cancer risk due to increased cell proliferation.
TP53 and BRCA2, tumor suppressor genes
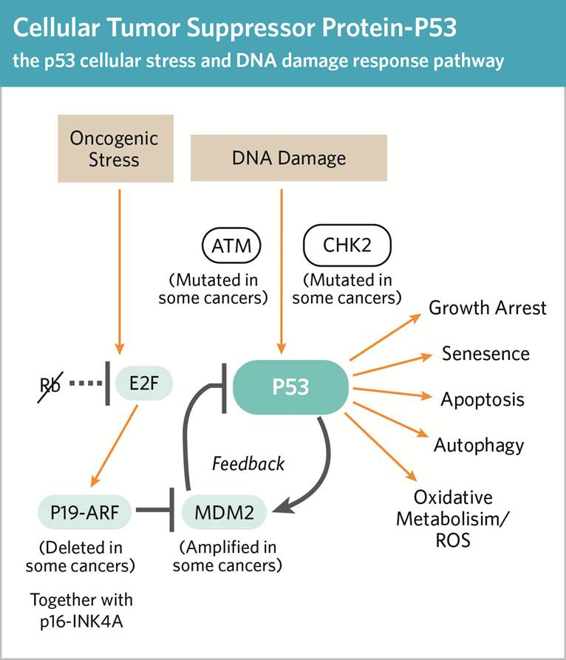
Tumor suppressor genes are cell cycle “brakes”, the protein products of which inhibit the cell cycle when something has gone wrong with DNA repair and synthesis. They can do this by blocking reproduction and/or initiating apoptosis in potentially cancerous cells. This capacity is lost with pathogenic variants. TP53 is a tumor suppressor gene that is nicknamed the “Guardian of the Genome”, as its protein product plays a role in removing cells with damaged DNA. The gene is located on chromosome 17 and consists of around 20000 nucleotides. Mutations on this gene have been associated with higher breast cancer rates, and with cases that are harder to treat. When a point mutation leads to a non-synonymous change in the amino acid sequence that results in a non-functional protein, then cells with mutations and/or damaged DNA can continue to propagate, leading to an increased risk of cancer.
Another tumor suppressor gene, BRCA2, is an example of a gene in which pathogenic variants are sometimes inherited. BRCA2 is located on chromosome 13 and consists of around 10000 base pairs across 27 coding sequences. The protein product of BRCA2 plays a role in repairing damaged DNA, and along with the gene BRCA1, it is sometimes nicknamed the “Caretaker of the Genome”. Similar to TP53, a mutation that results in a non-functional protein product can lead to an increased risk of cancer, since damaged DNA is not being repaired.
Cell Biology
- List the major levels of biological organization in multicellular organisms and explain why cancer is an inherent risk to all multicellular life
- Describe the major steps of the cell cycle, and explain why it must be carefully regulated to avoid cancer
Multicellular organization and cancer
Cells are the smallest individual units of life. The evolution of multicellularity allowed for the division of labor (e.g. movement, digestion, reproduction etc) between different cell/tissue types. However, multicellular life also has an inherent risk of cancer: Under certain conditions, individual cells may be selected to selfishly replicate at the expense of the organism. Cancers result from an accumulation of genetic mutations and can disrupt the regulatory processes within a cell cycle. Cells become self-interested, dividing on their own schedule, out of sync with others that surround them. They “cheat” the cellular system in which they reside, acting more like single celled organisms. They often start to look different from other cells as well. They outpace the reproduction of normal cells and can form a mass called a neoplasm. A neoplasm can progress to become tumor. Some tumors are harmless, benign, while others are malignant or cancerous. The progression from a harmless to cancerous tumor primarily depends on the function of enzymes and proteins that 1) regulate cell growth and division or 2) ensure faithful apoptosis and DNA synthesis. In breast cancer, the former includes protein receptors for epidermal growth factor (HER2) and the latter includes proteins involved in apoptosis (TP53) and DNA repair (BRCA2). The three genes corresponding to these proteins are discussed at some length in the Genetics module.
DNA synthesis, mitosis, and the HER2 & ESR1 proteins
Living cells need to occasionally replicate in order to keep tissues functioning and healthy. Cellular replication involves three major processes: DNA replication, mitosis, and cytokinesis. Before a cell can replicate, it must first synthesize new DNA. During this process, the strands of the double helix separate, each becoming a template for synthesis of a new strand. This is considered semi-conservative replication, since each progeny strand is directly derived from the parent molecule. During the synthesis of new DNA, mistakes in the nucleotide base sequence (i.e. mutations, discussed above) can occur. Sometimes these mistakes are dealt with via apoptosis or DNA repair (discussed below), but often they become part of the next generation of cells in our body. In fact, all of the somatic cells in adult bodies represent a “patchwork” of various mutations, sometimes referred to as somatic mosaicism. It is estimated that most tumors contain around 1000 to 20000 point mutations and hundreds of insertions, deletions, and rearrangements (Martincorena & Campbell, 2015).
There are a variety of proteins that are expressed in order to promote rapid cellular growth and replication. In some cases, this process is necessary to sustain healthy tissues (e.g. when there is damage). However, when these proteins are overexpressed, cells can begin to replicate more than they need to. For example, when an extracellular growth factor binds to a growth factor receptor (a protein product of HER2), a series of events leads to the promotion of cellular growth and replication. Therefore, any genetic change that leads to an overexpression of these membrane receptors can increase the rate of cell proliferation and ultimately lead to cancer.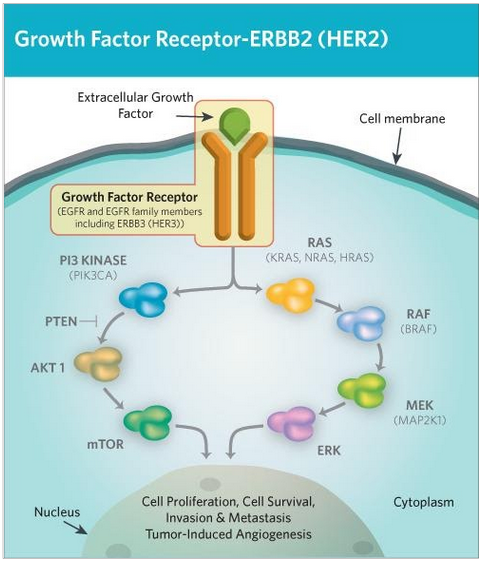
ESR1 is an estrogen receptor that consists of 595 amino acids. When bound to estrogen, travels to the nucleus where it increases the transcription of many genes that increase the rate of cell growth and replication. It is expressed in cells throughout the body, but it specifically fosters cell reproduction associated with the menstrual cycle, including the growth of breast tissue. The differences in estrogen levels between the sexes partially contributes to explain the increased risk of breast cancer in women compared to men.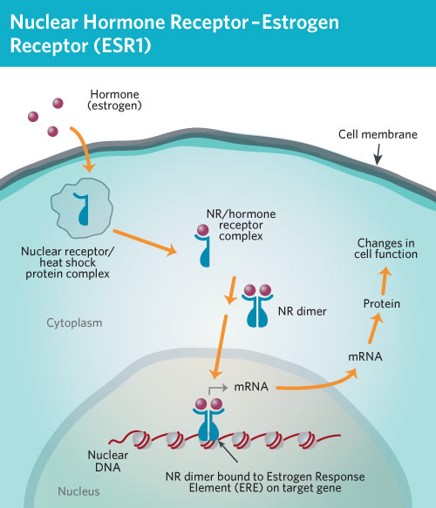
Apoptosis and the TP53 protein
Apoptosis is cell self-destruction that happens often during tissue development. Apoptosis is an important cellular process in the body as it gets rid of cells that have the potential to become cancerous (e.g. cells with numerous mutations and/or damaged DNA). Triggers can be external messages that bind to corresponding cell receptors on the cell membrane or by internal messages, generated by stress (eg heat, radiation, starvation, etc). One internal stressor is accumulation of errors in DNA synthesis sensed by checkpoint proteins such as p53 (the protein product of TP53). Once a cell gets and processes the signal to self-destruct, it undergoes a series of biochemical and morphological changes.

- A group of enzymes, caspases, activate the destruction of cellular organelles and the degradation of mRNA
- The cell shrinks as the cytoskeleton collapses: cell contents crowd together.
- The cell membrane folds in on itself and loses its structural integrity.
- In the nucleus, chromatin clumps form as DNA fragments.
- The cell collapses in on itself.
- The cell breaks apart, forming apoptotic fragments, which are cleared out by special cells called phagocytes.
There are both pro-apoptotic and anti-apoptotic proteins that regulate its occurrence. For example, p53 is a key pro-apoptotic factor. When released to the cytoplasm, p53 activates the cascade of caspases that lead to cell suicide. Other proteins, such as MDM2 inhibit pathways leading to cell death. Overexpression of these proteins can affect cell self-destruction by suppressing p53 and subsequent caspase cascades. Cancer is often associated with an increase in the pro- compared to the anti-apoptotic proteins. This imbalance allows these cells to proliferate, carrying their stressors and errors to the next generation of cells.
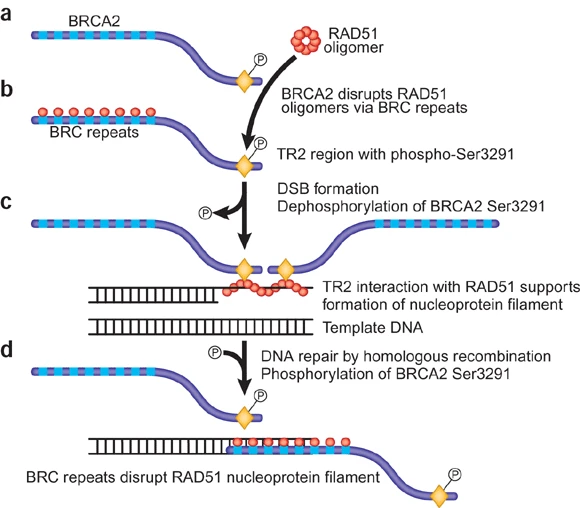
DNA Repair and the BRCA2 Protein
Evolution
The evolutionary puzzle of cancer: Why does breast cancer persist?
If breast cancer can often result in early mortality, then why is it so common throughout human populations? If natural selection tends to lead to increased fitness of the individuals within populations over time, then wouldn’t we expect harmful gene variants such as dysfunctional copies of BRCA1/2 to be selected out of the population? These questions can be addressed by two broad concepts: Firstly, natural selection is only one of five evolutionary mechanisms. Along with selection, these other four mechanisms (mutation, drift, gene flow, and sexual selection), are also constantly impacting the frequency of alleles within populations. Secondly, natural selection is often constrained by a variety of factors. For example, rapidly changing environments can often outpace natural selection. The sections below discuss how these evolutionary mechanisms and constraints can lead to the prevalence of breast cancer within human populations.
Antagonistic pleiotropy and trade-offs
An evolutionary trade-off refers to a situation in which evolution cannot optimize the fitness associated with a specific trait without compromising the fitness associated with another. One common source of trade-offs is antagonistic pleiotropy. Pleiotropy refers to when a single gene influences two or more phenotypic traits, and antagonistic refers to the fact that these two phenotypes oppose each other in terms of their effects on an individual’s evolutionary fitness (i.e. one positive effect, and one negative effect). Antagonistic pleiotropy usually occurs in a life-history context, where a gene variant that has positive fitness effect early in life (i.e. leading up to or during a reproductive age) has a negative effect later in life (i.e. post-reproductive age). Natural selection often favors these variants because they improve fitness during a reproductive age, even though there a cost later in life (i.e. a trade-off).
There is some evidence to suggest that BRCA1/2 variants in humans represents a case of antagonistic pleiotropy, where the same variants that increase breast cancer risk also appear to be linked to increased reproductive success. Specifically, historical family data has uncovered trends in various metrics of fertility between carriers and non-carriers of BRCA1/2 variants. Smith et al. (2011) reported that women with BRCA1/2 mutations tended to bear more offspring over their lifetime compared to women without BRCA1/2 mutations, but they also had an increased risk of mortality at a post-reproductive age. Mutation carriers also tended to have shorter intervals between births and ended childbearing at a later age than controls. In a similar study, Kwiatkowski et al. (2015) report that BRCA 1/2 mutation carriers were less likely to bear no children in their lifetime, and tended to have more children overall. Although these data are intriguing, it is worth noting that contradictory evidence suggests these trends may not be generalizable across populations.
Founder effects and population genetics
Environmental mismatches
A final explanation for high rates of breast cancer in human populations is that we live in vastly different environments compared to a majority of ancestral humans. Natural selection is a process that occurs over many generations, but human environments can change rapidly, especially since the recent industrial and technological revolutions. In general, modern humans have different diets, reproductive patterns, and lifestyles compared to ancestral humans. The asymmetry between ancestral and modern conditions is called an environmental mismatch.
One element that differs quite dramatically between many modern and ancestral human populations are typical reproductive patterns. In many modern cultures, birth rates have drastically declined. In the USA, the average woman has over 400 menstrual cycles compared to around 100 in a culture in Africa without birth control and lower rates of breast cancer (Strassmann, 1999). The mechanism behind this is likely related to increased hormone exposure, as estrogen receptors in breast tissue initiate cell division, and exposure to compounds that mimic estrogens have been implicated in higher rates of breast cancer. As a result, breast cancer estimated to be 10x higher in women in the USA compared to hunter-gatherers (Eaton et al., 1994). Furthermore, a meta-analysis identified the number of pregnancies as being a significant factor associated with reduced risk of breast cancer (Ewertz et al., 1990). Finally, across cultures, there is a strong association between breast cancer risk and average levels of progesterone during menstruation (Jasienska & Thune, 2001), suggesting a relationship between lifestyle factors, hormones, and the risk of breast cancer.
Aside from reproductive patterns, there are many other elements of typical modern environments that contribute to increased rates of breast cancer. For example, about one third of all cancer can be attributed in part to tobacco use, and another third to obesity. Another major risk factor associated with many cancers is age. On average, humans in modern environments have longer lifespans, meaning the risk of cancer within one’s lifetime is also higher.
Treatment and Ethics
Determining treatment
When a lineage of cells that is dividing uncontrollably evolves, they sometimes progress into a tumor. Some tumors threaten the function of one or more body organs because these cells don’t share cellular resources. These cells need to be removed, killed, or otherwise managed so they don’t pose a threat to body organs or systems. Tumors are given a “stage” classification, based on an assessment of three factors:
1) Size of primary tumor (T)
2) Number of lymph nodes with cancerous cells (N)
3) Whether the cancer is metastatic (M)
The assessment of the tumor TNM will lead to a stage being assigned. Determining the most appropriate treatment depends on the stage of the cancer.
Stage 0: In situ. Tumor is small and localized. Typically treated with surgery.
Stage 1: Early-stage cancer. Tumor is still small and has not spread to surrounding tissues. Often treated via surgery.
Stage 2-3: Early-to-late locally advanced. Tumor has grown to affect surrounding tissue. May also be in lymph nodes. Often treated with surgery, radiation, and chemotherapy.
Stage 4: Metastatic cancer. The cancer has spread to affect more than one part of the body. Often requires aggressive treatment with surgery, radiation, and chemotherapy. Can become terminal.
Traditional cancer treatment
Traditional approaches to cancer treatment typically involve attempting to outright eliminate all cancerous cells from the body. Often, these approaches are quite aggressive and can harm healthy cells in the process. Broadly, there are five ways cancer can be eliminated, and it is typical for more than one approach to be used.
Surgery: Depending on the size, location and nature of a tumor, a lumpectomy may be performed. Usually, the tumor and some of the surrounding tissue is removed. Other times, breast cancer may require a more radical procedure called a mastectomy, where one or both breasts are removed. Some people chose preventative mastectomies based on family history of breast cancer.
Radiation therapy: Radiation therapy works by introducing an extremely mutagenic agent (radiation) to tumor cells. The radiation damages tumor cell DNA so severely that apoptosis is initiated, but it also damages any non-cancerous cells in the vicinity of the tumorous cells. Radiation therapy can be external or internal. In internal therapy, a calibrated dose of X-ray is directed at the tumor. To lower the risk of death to normal cells, these treatments are spread out over time and directed at the tumor from different directions. Since the tumor cells are always the focal point of the X-ray radiation, the repeated exposure typically kills them. Normal cells in the surrounding tissue are only intermittently exposed to the radiation and those tissues thus have time to heal between treatments (i.e. because of the different directions of the radiation from one treatment to the next). Some radiation therapy is delivered internally, most commonly as an implanted “seed” placed in, on or near the tumor. Cancers amenable to this treatment include cervical, prostate, eye and breast. Once an applicator is installed and a seed inserted, a low dose may be in place for just a few days, usually in a hospital setting. Some higher dose treatments apply the implant for very short intervals over several days. Permanent seed implants are not removed, but over time the radiation weakens to nothing.
Chemotherapy: Chemotherapy is a broad term that simply refers to a chemical treatment of cancer. Chemotherapy typically introducing a drug that will target fast replicating cells. If replicating cells are damaged, and initiate apoptosis instead of dividing, then cancerous cells will not proliferate. For tumors distributed throughout the body chemotherapy may be the best course of action for large primary and small, even unseen, secondary tumors. Chemotherapeutic agents can be administered orally or by infusion. Since they can also affect healthy cells, side effects are common and sometimes severe. Many chemotherapeutic drugs group work by damaging DNA or interfering with its replication or transcription, which tends to result in apoptosis. These drugs include fluoro-uracil, which disrupts base-pairing, platinum-based compounds, which disrupt DNA replication (commonly used to deal with BRCA1/2 mutations), and actinomycin D which blocks transcription to RNA. Oher chemotherapeutic drugs can interfere with mitotic spindle fibers that separate and move chromosomes. These drugs prevent cell division and include taxanes, which are proteins that bind and inhibit the function of microtubules, and vinblastine and vincristine, which are proteins that bind to tubulin, the subunit of microtubules.
Hormone therapy: Hormone therapy is a drug-based treatment that is designed interfere with hormone-mediated cell growth. In the case of breast cancer, estrogens are known promoters of cell reproduction in both normal and cancerous cells in breast tissue. The drug tamoxifen acts like estrogen and binds to the estrogen receptor, ESR1, blocking it from promoting cell reproduction. When tamoxifen binds to ESR1, it inhibits its ability to stimulate cell growth. Another strategy is to prevent estrogen from being made in the first place. One pathway to estrogen synthesis requires an enzyme, aromatase, which converts androgens into estrogens. Aromatase inhibitors decrease the level of circulating estrogens, lowering its overall stimulatory effect.
Immunotherapy: The immune system can identify and destroy oncogenic viruses and other pathogens that promote cell growth (these are rare). In terms of cancer, a sub-set of immune system cells called cytotoxic T cells and NK cells, target and kill the host’s own cells that have evolved to the point where they are identified as invader cells (instead of your regular body cells). However, cancer cells are often able to invade this mechanism of defense (see Ecology in Cancer Across the Tree of Life Case). Knowledge of the immune system, and its relationship to cancer cells, has led to some targeted cancer therapies, used primarily in advanced cancers with known cell biology characteristics/markers. For example, in cancers with HER2 mutations, monoclonal antibodies can bind to mutant HER2 receptors and turn them off. This slows down the cell growth signaling system. Vaccines can up-regulate an immune response to antigens associated with tumor cells. T-cell transfer therapy: taking most active tumor fighting T cells from a patient, culturing them and putting back in patient, Immune system modulators: proteins that boost the immune response, such as interferons and interleukins.
An evolutionary approach to treatment
Cancer treatments often lose their effectiveness over time. This happens because treatments that seek to eliminate cancer cells often create a natural selection pressure on the population of cells. If they are not effective at killing ALL cancerous cells, then those that are resistant to the treatment survive and reproduce. As a result, the subsequent tumor growth becomes resistant to the treatment that was previously effective. Radiation and chemotherapy particularly present a strong selective pressure. This is because although most cancerous cells are killed, some are able to resist the specific treatment due to randomly occurring mutations and go on to reproduce. When a round of cancer treatment concludes, the resistant cells divide aggressively resulting in new tumor growth. However, it is generally assumed that there is a metabolic cost for being resistant. For example, some resistant cells need to actively actually pump out the chemotherapy molecules to escape their impacts.
While this can be catastrophic for the effectiveness traditional cancer treatment, it opens the door to an approach called adaptive therapy. Adaptive therapy is an evolution-based strategy that aims to manage the tumor by creating a balance of resistant and sensitive cells rather than trying to extinguish the whole thing with chemotherapy. Basically, chemotherapy is administered so that the tumor shrinks to some predetermined size, allowing both treatment-sensitive and treatment-resistant cells to survive. The tumor would grow again, with sensitive clones growing keeping up with the resistant ones. At a certain size, the treatment regime would be repeated until the tumor receded back to a base level size. This back-and-forth would be repeated as long as the patient was alive. The idea is to manage the tumor, rather than beat it into submission, a futile approach. This treatment can provide patients with respite from being poisoned by aggressive treatments, leading to better quality of life. This approach has been used in small studies of prostate cancer patients with very encouraging results. The patients reported feeling better and they lived longer than is the case with “standard” chemotherapy treatment. To summarize, adaptive therapy involves applying principles across biological sub-disciplines (cell biology, genetics, ecology and evolution) to adapt treatment to control the “beast” at hand, rather than trying to outright destroy it.
An evolutionary perspective of cancer has also led to advancements in personalized cancer treatments. This is because an evolutionary perspective recognizes that each cancer, and the body in which it resides, is unique. The first step of personalized treatment is to sequence the genome of multiple samples of a tumor to assess the probable range of gene expression in a tumor. For example, what are the proteins are the clones producing, and what is the role of each in the tumor? Characterizing the patchwork of cells in a tumor allows oncologists to predict a strategy of potential personalized treatments, such as chemotherapy for targeted genetic signatures, testing chemo in masses of cells cultured from a patient to determine specific drugs, and/or using CRISPR techniques to cut out cancer alleles.
Ethics
Treating breast cancer can be really expensive, so considering equitable access to treatment is an important factor when designing treatment strategies. Medical science, along with agriculture, has steadily allowed individual humans to live longer and have access to a higher quality of life. This is particularly true in wealthy first-world societies. New therapies, particularly chemotherapy for cancer, are regularly being developed at a high cost. Some of them are marginally and/or variably effective, prolonging misery yet not significantly prolonging life. This has been termed, not affectionately, as the oncology/industrial complex, and raises questions regarding the justification of some of these treatments. In a 2016 study group of 8360 women (USA) with cancer stages 0 – IV, the cost of treatment for the first year, per person, was:
Stage 0 – $60,637
Stage I/II – $82,121
Stage III – $129,387
Stage IV – $134,682
For many Americans without insurance, access to this level of care is unaffordable, and even those with private insurance often face a high co-pay cost. The high cost, along with unequal access to care raises a variety of ethical questions.