Bacteria Citrate Use
Bacteria are excellent model organisms for studying evolution. They are easy to grow, maintain, and handle under very controlled conditions. Even more importantly for evolution research, they can grow to very large population sizes in small culture flasks, and reproduce very rapidly. These characteristics mean that experimental populations can have a steady supply of variation for natural selection to work upon, and these populations can evolve quickly enough for evolution to be studied in action. Finally, unlike mammals, bacteria can be frozen without being killed, so samples can be taken of evolving populations that can be frozen regularly, and revived to discover how evolution worked. One of the most studied bacteria is E. coli, which is found in the gut of most warm-blooded animals.
In 1988, Dr. Richard Lenski started the Long-Term Evolution Experiment (LTEE), when he founded 12 populations of E. coli from a single clone. Every day 1% of each population is transferred to a flask containing a fresh volume of a nutrient broth containing a small amount of glucose for the bacteria to use as food. Each day the populations go through about 6.67 generations, and the twelve have each evolved for more than 60,000 generations over the course of the experiment so far. Samples of each population are frozen every 500 generations, meaning that Dr. Lenski has a complete frozen fossil record of the evolution of each population from which he can revive bacteria at any time to study their evolution.
The nutrient broth also contains a large amount of citrate, which is included to help the bacteria take up the iron they need to grow. This citrate could be a second food source for the bacteria, but one of the defining characteristics of E. coli as a species is its inability to grow on citrate when oxygen is present. However, after ~31,000 bacterial generations, one of the populations evolved the capacity to do just that. The evolution of this trait, called Cit+, is exceptionally rare. Indeed, spontaneous evolution of a Cit+ E. coli was reported only once in the entire 20th century!
Cell Biology
After ~33,000 generations, or 16 years into Dr. Lenski’s experiment, population #9 suddenly became much larger. Dr. Lenski’s students discovered that a lineage in this population evolved the ability to grow on the citrate in the bacteria’s nutrient broth at about the 31,000 generation time point.
The Use of Citrate
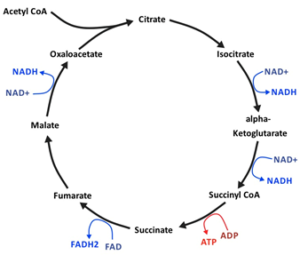 E. coli is normally unble to grow on citrate when oxygen is present. However, it has a citric acid cycle, meaning that it can metabolize citrate during aerobic growth on other substances. E. coli therefore has the cellular machinery to grow on citrate if it can transport it into the cell. E. coli can grow on citrate when no oxygen is present through anaerobic processes. E. coli is able to bring citrate into the cell under these conditions because it has a gene called citT that encodes a transmembrane citrate-succinate antiporter. However, citT is part of an operon containing genes needed for citrate fermentation that is only turned on in the absence of oxygen.
E. coli is normally unble to grow on citrate when oxygen is present. However, it has a citric acid cycle, meaning that it can metabolize citrate during aerobic growth on other substances. E. coli therefore has the cellular machinery to grow on citrate if it can transport it into the cell. E. coli can grow on citrate when no oxygen is present through anaerobic processes. E. coli is able to bring citrate into the cell under these conditions because it has a gene called citT that encodes a transmembrane citrate-succinate antiporter. However, citT is part of an operon containing genes needed for citrate fermentation that is only turned on in the absence of oxygen.
Citrate Transport
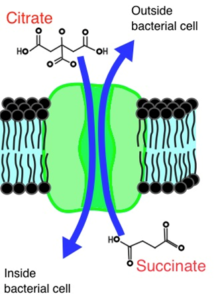 CitT is a transmembrane antiporter than can bring a broad array of di- and tri-carboxylic acids into the cell in exchange for other di- and tri-carboxylic acids. This exchange is a form of secondary active transport in which the energy for importing one substrate is provided by the export of the other substrate down an electrochemical gradient from high concentration to low concentration, with no need for ATP to drive the process. CitT principally functions as a citrate-succinate antiporter, which makes it ideal for citrate processing, in which succinate is an end product. In the Cit+ lineage in LTEE population #9, a complex mutation allows the cells to express the CitT transporter in the presence of oxygen (See the molecular genetics section) and thereby access the citrate resource. When citrate is pumped into a Cit+ cell, it enters the citrate acid cycle directly, as opposed to citrate synthesized from oxaloacetate and acetyl-CoA during glucose metabolism. The citrate is then converted to succinate through the citric acid cycle’s reactions, producing energy in the process. The succinate is then pumped out into the medium in exchange for more citrate.
CitT is a transmembrane antiporter than can bring a broad array of di- and tri-carboxylic acids into the cell in exchange for other di- and tri-carboxylic acids. This exchange is a form of secondary active transport in which the energy for importing one substrate is provided by the export of the other substrate down an electrochemical gradient from high concentration to low concentration, with no need for ATP to drive the process. CitT principally functions as a citrate-succinate antiporter, which makes it ideal for citrate processing, in which succinate is an end product. In the Cit+ lineage in LTEE population #9, a complex mutation allows the cells to express the CitT transporter in the presence of oxygen (See the molecular genetics section) and thereby access the citrate resource. When citrate is pumped into a Cit+ cell, it enters the citrate acid cycle directly, as opposed to citrate synthesized from oxaloacetate and acetyl-CoA during glucose metabolism. The citrate is then converted to succinate through the citric acid cycle’s reactions, producing energy in the process. The succinate is then pumped out into the medium in exchange for more citrate.
Molecular Genetics
Bacterial genes involved in the same process are often clustered together in the same location on the chromosome, where they are under the control of the same promoter. This ensures that all of the genes are expressed at the same time, and under the conditions in which they are all needed. The genes encoding the proteins needed for fermentation of citrate are all clustered together in the cit operon that is controlled by a promoter that is only active when no oxygen is present.
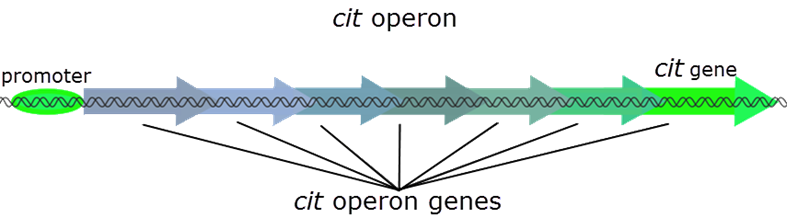
A Duplication Mutation and a New Arrangement
Whole-genome sequencing has shown that the cells in the Cit+ lineage from LTEE population #9 all have a duplication mutation that overlaps the cit operon. The duplicated segment is 2933 bp long, and extends from the 3’ end of the citG gene (in the purple arrow in the diagram) to the 5’ end of the rnk gene (the blue arrow).
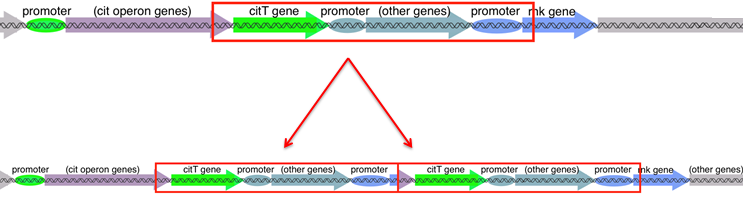
The duplication contains citT as well as the upstream regulatory region and promoter of rnk. The duplication is tandem, meaning that the new copy is immediately adjacent, head-to-tail with the original copy. Because of this structure, the new copy of citT ends up being downstream and under the control of a copy of rnk’s promoter, rewiring these genetic elements and creating the new rnk-citT regulatory module. The rnk gene is normally expressed during aerobic growth, meaning that its promoter supports rnk transcription when oxygen is present. The rnk-citT module therefore leads to expression of CitT during aerobic growth, and provides access to the large citrate resource.
Ecology and Phylogenetics
The new Cit+ lineage in population #9 arose ~31,000 generations into the LTEE, and became dominant ~33,000 generations. However, the Cit+ lineage did not completely sweep the population. Instead, a small subpopulation of Cit– cells that could not use the citrate coexisted with the Cit+ subpopulation for over 10,000 generations.

Cit- and Cit+ Coexistence
How were Cit– and Cit+ able to coexist? For two or more types of organisms to coexist in an environment as part of a diverse ecology, they have to occupy different niches. For example, different organisms could coexist if they used different resources, or if they used the same resources, but to different degrees. In the case of Cit+ and Cit–, the answer involves a bit of both of these possibilities. The Cit+ cells, of course, had exclusive access to the citrate resource, but both Cit+ and Cit– could use the glucose resource. However, in the course of evolving to be better at growing on citrate, the Cit+ lineage lost some of its ability to compete with Cit– for glucose, giving Cit– a glucose growth advantage.
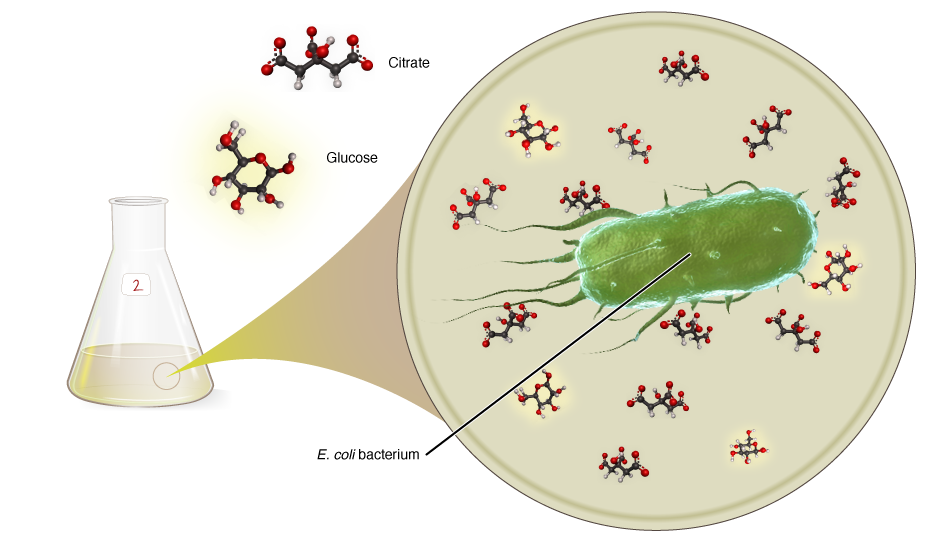
The difference in ability to compete for glucose is not the whole story, though. As discussed in the Cell Biology section, the CitT antiporter that Cit+ uses to bring citrate into the cell works by pumping citrate into the cell while pumping succinate out of the cell. This means that the Cit+ cells produce a pool of succinate in the broth while it grows on citrate. This succinate, while not as energy-rich as either glucose or citrate, is a third resource that the bacteria can grow on. As with the glucose, both cell types can grow on succinate, but Cit– appears to have had some advantage in competition for it. The evolution of Cit+ therefore turned the broth that previously had one resource, glucose, into one that contains three: glucose, citrate, and succinate. Cit+ can eat all three, while Cit– could eat glucose and succinate, and because it can compete a bit better for them than Cit+, Cit– was able to persist in the population despite being a tiny minority. Did Cit+ evolve to compete better for glucose and succinate, nearly driving Cit– out of the population, or was it more random? Research is currently under way to figure out that question.
Phylogenetics
Phylogenetics is the study of the evolutionary relationships between groups of organisms. Those relationships are inferred by comparing sets of traits of the organisms under study. Shared traits indicate shared ancestry. Those traits that are not shared might have been lost by one or more lineages after they diverged, or they might have evolved after a lineage diverged, in which case they are referred to as derived. While many traits have been used to infer evolutionary relationships, DNA and protein sequences have come to be the traits most commonly used.
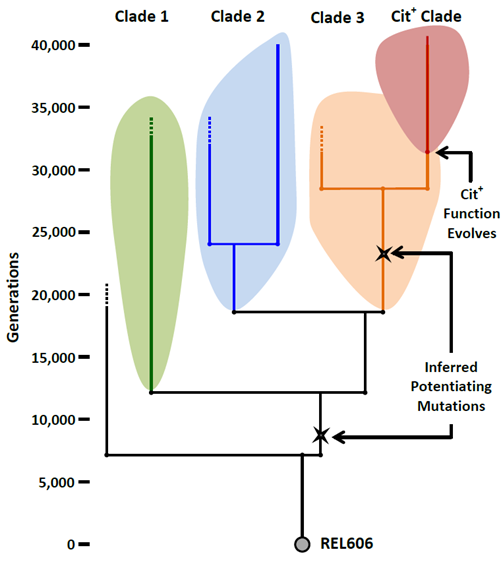
Once evolutionary relationships have been inferred, they are usually depicted visually with phylogenetic trees. In a phylogenetic tree, the organisms from which traits were compared are represented as branch tips, and inferred common ancestors are represented as the nodes from which branches emerge. A clade is a common ancestor and all of its descendants. On a phylogenetic tree, a clade is a node and all of the branches that emerge from it, meaning that it is monophyletic. Because all organisms share a common ancestor at some point, a phylogenetic tree can be thought of as a series of nested clades, with the most inclusive clade being the entire tree.
The phylogeny of LTEE population #9 was determined using data from the sequencing of the whole genomes of clones isolated from various time points in the frozen fossil record. The phylogeny shows that the population was heterogeneous for most of its history even before Cit+ evolved. At least three major clades coexisted in the population from before 20,000 generations until after 33,000 generations. As discussed earlier, such coexistence suggests that the different clades were occupying slightly different niches. What these niches were and what sorts of ecological interactions took place between the three clades is the subject of ongoing research. The phylogeny also shows that all Cit+ clones belong to a single lineage that diverged from Clade 3. The original Cit+ clone and all of the Cit+ cells that descend from it are now a new clade, the Cit+ Clade.
